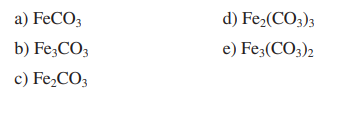aqueous iron chloride and sodium carbonate solution yields aqueous sodium chloride and a precipitate of iron - Brainly.in

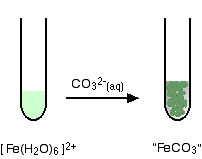
Fe(III) mobilisation by carbonate in low temperature environments: Study of the solubility of ferrihydrite in carbonate media and the formation of Fe( III) carbonate complexes - ScienceDirect

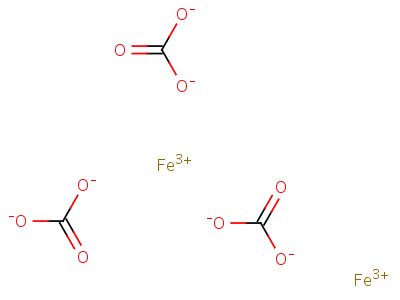
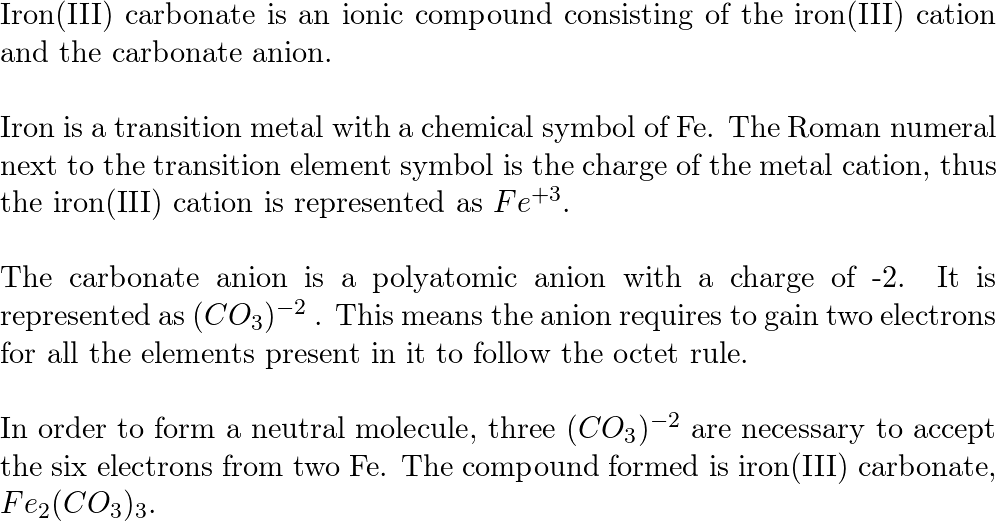
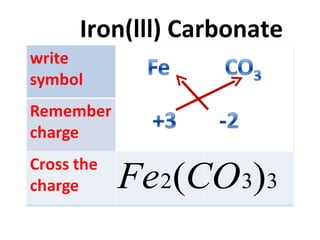
in Valency of Iron in FeCl, is ...........and in FeCl, it is... g) Formula of iron (III) carbonate is . . .